Best Describes a Solution in Which Alcohol Is the Solvent
C Properties of a solution that depends on the temperature change of a solution. Perhaps the most common solvent in everyday life is water.
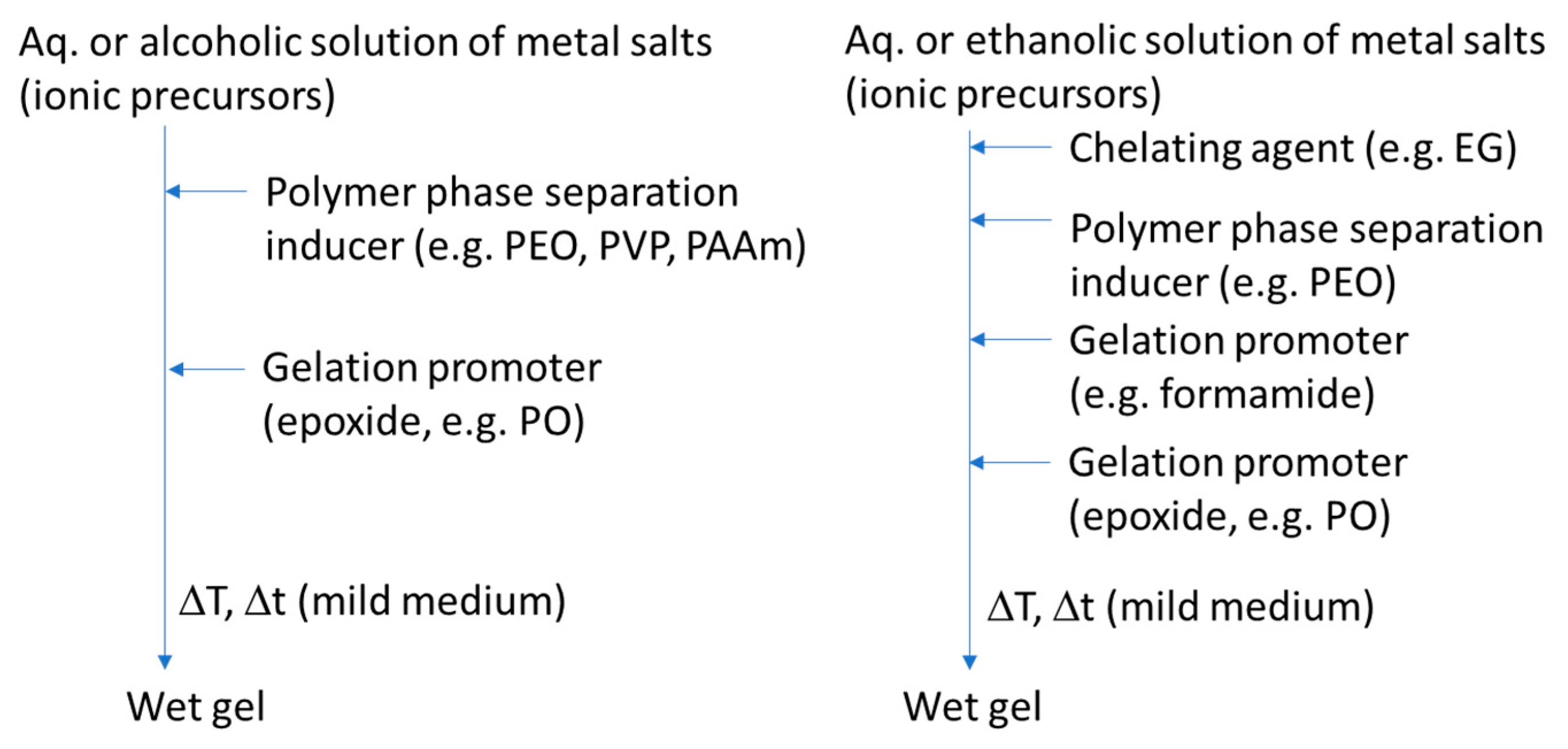
Materials Free Full Text Macroporosity Control By Phase Separation In Sol Gel Derived Monoliths And Microspheres Html
A solvent is a liquid that dissolves a solute.
. It contains a solute and solvent B. Significantly the comments are not factual. Organic solvents always contain the element carbon while inorganic solvents do not.
Which best describes the parts of a solution. In addition these solvents molecules will be able to readily donate protons H to solutes mostly via hydrogen bonding. Which compound is a waste product of cellular metabolism.
Since both ethanolwater and solution are in the same phase and ethanol is in greater proportion it will be called as the solvent. A popular example of. The solvent is ethanol.
Which statement best describes the formation of a solution. If isopropanol extraction will be OK for a given soluble in alcohol. The H of the solution will increase.
Which of the following best describes a solution in which alcohol is the solvent. 3 bronze badges. The solvent is the greater part the solute is the lesser part d.
If the solvent contains a labile H then they are classified as such. What part of the solution is alcohol. Instead they seem to reflect a neo-prohibitionist effort to stigmatize alcohol.
The scattering of light by solvent in a solution the scattering of light by solutes in a solution the scattering of light by particles in a mixture 2 See answers Advertisement. The solute is the solid part the solvent is the liquid part c. The relationship between solute solvent and solution is.
The only true solutions are formed when water dissolves a polar solute. Its components are evenly distributed in the solution C. 2 A solvent may contain either organic or inorganic chemicals.
Which should be the best solvent in terms of amount of solute dissolved in given volume of solvent and why. The only true solutions are formed when water dissolves a nonpolar solute. A solution that has a pH of 68.
Which of the following statements best describes the phrase like dissolves like. Astonishingly government officials of today made them. Solute and solvent are two of the components of a solutionThe solute is the substance solid liquid or gas that dissolves in the solvent to produce a homogeneous.
So the assertion that alcohol is a solvent is an effort to stigmatize alcoholic beverages. Considering the definition of solute solvent and solution the correct answer is option 2. Which best describes the Tyndall effect.
That would never happen. Of course the notion depends on context too. A solvent and a solute with similar intermolecular forces will easily make a solution.
That would be an acid and a base that would immediately turn back to water. A large amount of solvent is dissolved in a larger amount of solute. A small amount of solvent is dissolved in a larger amount of solute.
After acid is added to the alcohol what is the general name of the first reaction intermedia. A small amount of solute is dissolved in a larger amount of solvent. Which of the following acts as a catalyst.
All these statements are very misleading at best. A Properties of a solution that depend on the concentration of solute particles in the solvent. Is there any a priori way to determine eg.
1 Choose which of the following best describes the term colligative property Group of answer choices. However the condition is simple for this type of solvent. 2 which of the following statements best describes a homogeneous solution A.
The solute is the greater part the solvent is the lesser part b. To prevent racemization To dissolve p-toluenesulfonic acid because it is insoluble in other solvents Because of their high boiling point To drive the equilibrium towards products 7 4 points What type of reaction best describes the second step. Non-halogenated solvent-water mixtures or non-halogenated solvents containing more than 20 water such as ethanol 80 Acetone toluene acetonitrile ethyl acetate heptane hexane and alcohol containing less than 20 water are examples.
The solute and solvent cannot be polar e. Which of the following best describes a solution in which water is the solvent. B All of these are correct.
A solute is dissolved in a solvent to form a solution. The most common solvent water is an example of an inorganic solvent. In chemistry a common rule for determining if a solvent will dissolve a given solute is like.
If I were studying a bunch of alcohols at different concentrations in water. Many other solvents are organic compounds such as benzene tetrachloroethylene or turpentine. A solution consists of a solute and a solvent.
You can still identify the components of the solution D. A solution requires at least two different components. Isopropanol ie isopropyl alcohol is a clear colorless bitter liquid commonly found in rubbing alcohol skin lotion hair tonics aftershave lotion denatured alcohol solvents cements cleaning products and de-icers.
6 TU 3 points In Fischer esterification reactions why is the alcohol used as a solvent that is in excess. The solvent is the component of a solution that is present in greater amount. Which of the following best describes a solution in which the alcohol is the solvent.
Which of the following best describes a solution in which alcohol is the solvent. In general which alcohols are suitableunsuitable for organic compound extraction eg. It is usually liquid 3 A small amount of alcohol is dissolved in water.
Intoxication may occur through ingestion or inhalation of vapors especially in infants. A solution might look like alcohol being the solvent that would take water the solute into solution making H X cations and O H X anions. A large amount of solute is dissolved in a smaller amount of solvent.
Sep 26 2016 at 700. The major difference between Aqueous and Tincture solutions is that in an Aqueous solution the solvent is water while in a Tincture solution the solvent is alcohol.

Factors Contributing To The Escalation Of Alcohol Consumption Sciencedirect

Pin On All Homemade Cleaners Etc

Ch104 Chapter 7 Solutions Chemistry

Is Alcohol Really A Solution Chemistry Stack Exchange

Pdf Solubility Of Butylated Hydroxytoluene Bht In Aqueous And Alcohol Solutions From 293 15 To 313 15 K

Alcohol Its Analysis In Blood And Breath For Forensic Purposes Impairment Effects And Acute Toxicity Jones 2019 Wires Forensic Science Wiley Online Library

I Just Love Aesop Product Design Http Www Aesop Com Aesop Skincare Skincare Branding Beauty Packaging

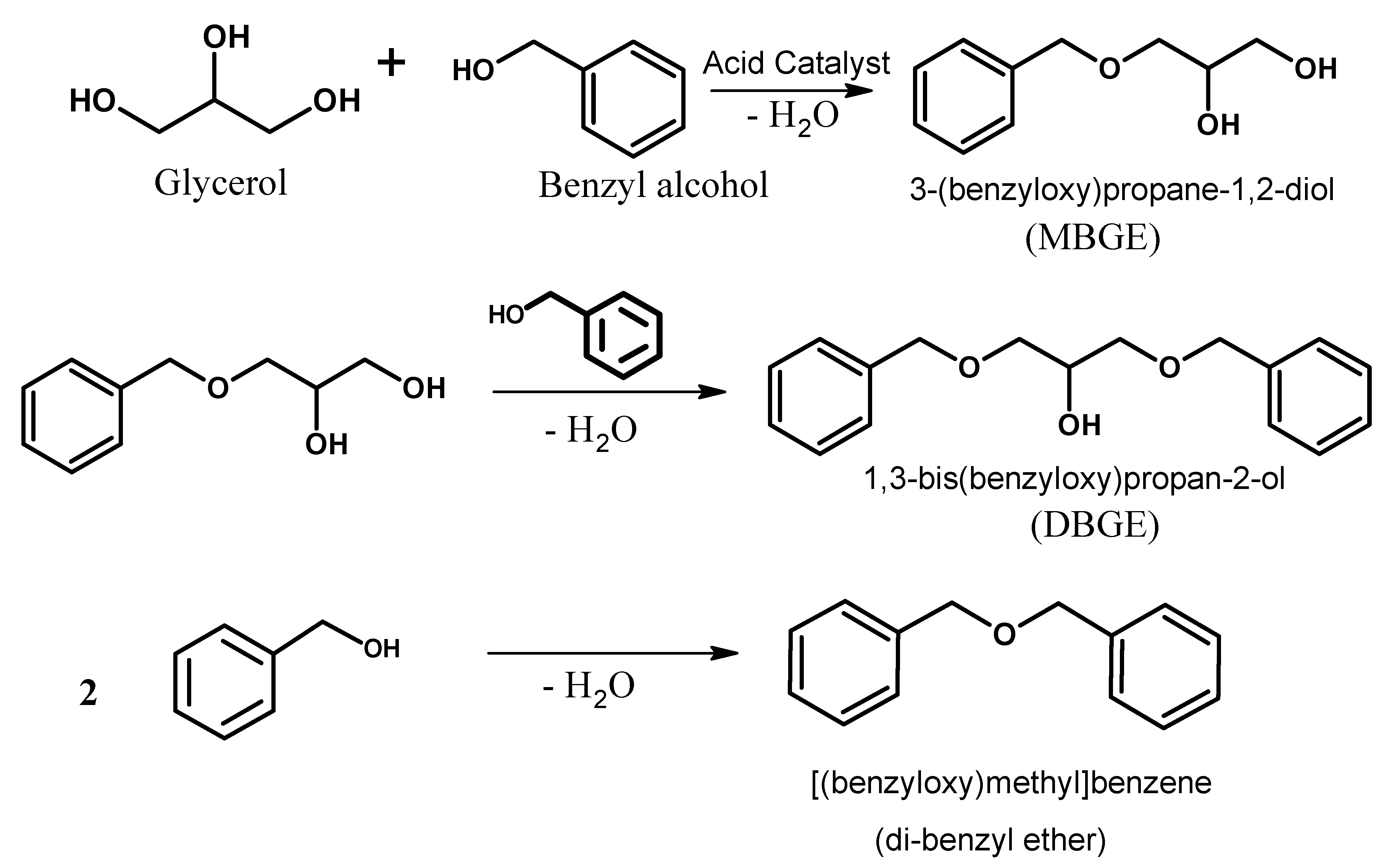
Catalysts Free Full Text Solvent Free Benzylation Of Glycerol By Benzyl Alcohol Using Heteropoly Acid Impregnated On K 10 Clay As Catalyst Html

Lesson Percentage Concentration Nagwa

Unstopping A Clogged Drain Naturally Cleaning Baking Soda Baking Soda Vinegar
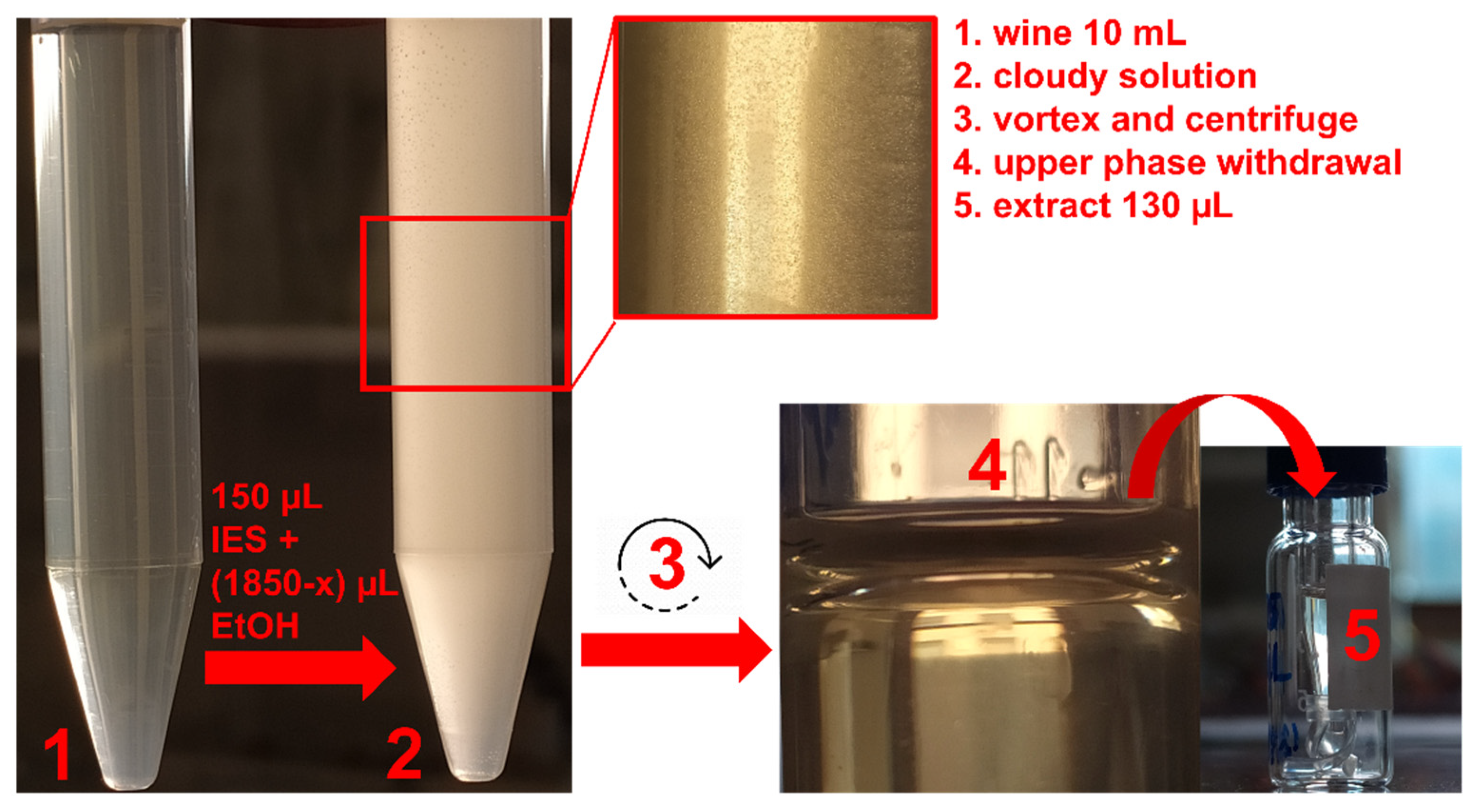
Molecules Free Full Text Hydrophobic Eutectic Solvent Based Dispersive Liquid Liquid Microextraction Applied To The Analysis Of Pesticides In Wine Html
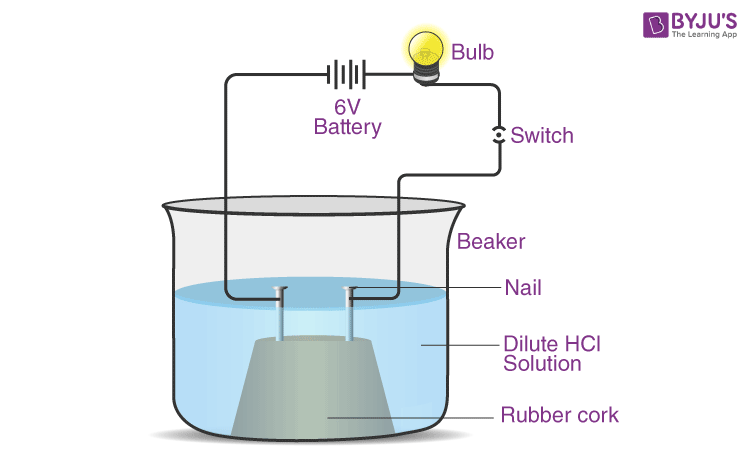
Compounds Such As Alcohols And Glucose Also Contain Hydrogen But Are Not Categorised As Acids Describe An Activity To Prove It Chemistry Q A

Ch104 Chapter 7 Solutions Chemistry

4 1 Solution Concentration And The Role Of Water As A Solvent Chemistry Libretexts

75 Clever Ways To Use Dish Soap That Save Time And Money Soap Dish Soap Hand Wash Dishes

Diy Natural Alcohol Free Toner For Oily Acne Prone Skin Alcohol Free Toner Diy Natural Products Alcohol Free

Compounds Such As Alcohols And Glucose Also Contain Hydrogen But Are Not Categorised As Acids Describe An Activity To Prove It Chemistry Q A

Yarabook Indian Social Network Social Network Free Advertising Alcohol

Comments
Post a Comment